Abstract
Introduction: The achievement of MRD negativity is a surrogate marker for better outcomes in MM. Our group recently showed that patients in CR but with persistent residual disease have an activated PD1/PDL1 axis (Paiva, Leukemia 2015), and this might be responsible for the failure to completely eradicate the residual myeloma cells.
Methods: In this phase II trial, 20 MM patients achieving at least VGPR but with persistent residual disease after 1 or 2 lines of therapy received the anti-PD1 MoAb Pembrolizumab as monotherapy at a dose of 200 mg q3w for 12 months. The primary aim of the study was to evaluate the ability of Pembrolizumab to upgrade responses. A secondary objective was to identify biological markers of response or resistance to Pembrolizumab. For this purpose, patient's immune system (B and T lymphocytes, NK cells, dendritic cells, monocytes and their respective subpopulations) was characterized in BM and PB by multiparameter flow cytometry and the PD1/PDL1 expression of these populations was also assessed. In addition, changes induced by Pembrolizumab on PB samples at C3D1 were evaluated. Statistical association with response categories was performed using the Mann-Whitney U exact test or the Kruskal-Wallis test followed by the Dunn's test when 2 and 3 groups were compared, respectively.
Results: Out of the 20 patients included, 18 with a median age of 63 (43-78) years are evaluable. Thirteen patients were included following 1st line of therapy (12 after HDT-ASCT) and 5 after 2nd line (3 after ASCT and 2 after MPV/LD combinations). At trial entry, 12 patients were in VGPR, 3 in CR and 3 in MRD+ sCR.
The median number of Pembrolizumab cycles was 6.5 (1-10). Four patients are not evaluable for efficacy due to the short follow up. Among the 14 evaluable patients, 3 (21%) upgraded their response: 2 patients in VGPR converted into sCR (at cycle 3 & 6, respectively) and 1 CR patient achieved an immunophenotypic CR (MRD-) (by cycle 7). In two additional patients (included in VGPR and sCR) there is an ongoing reduction of the FLC and MRD levels, respectively, but cannot be considered yet as an upgrade in the response category. No statistically significant association was found between the response to Pembrolizumab and status at inclusion, number of lines or prior ASCT. Of note, 5 patients have already progressed on cycles 3, 5, 6, 7 and 8 respectively.
Pembrolizumab was well tolerated with no related AE. Only one SAE has been reported; an treatment-unrelated femoral supracondylar fracture.
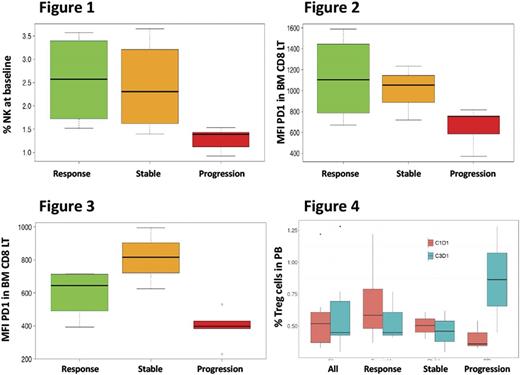
For the investigation of immune biomarkers predicting efficacy, patients were grouped in three categories: responders (n=5); progressed (n=5) and stable (n=4). First, the potential influence of baseline patient's immune status on treatment response was analyzed. The only differential feature in responders vs non-responders, was a higher percentage of gamma-delta T lymphocytes in BM (p=0.03). By contrast, patients that progressed had a clear differential immuno-profiling, including: 1) a significantly lower percentage of NK cells in BM (1.78% vs 2.49% in the remaining patients. p=0.011 Figure 1), together with slightly lower percentages of circulating and adaptive NK subsets in PB; 2) a lower PD1 expression in CD8+ T-cells both in BM (p=0.07) and PB (p=0.02) (Figures 2 & 3) particularly evident in the effector memory subset;
With regards to changes induced by Pembrolizumab in PB samples obtained at C3D1, a global increase in memory B cells (from 0.07% to 0.19%, p=0.02) was noted. Interestingly, whereas T regs decreased from 0.69% to 0.54% in responders, this population increased to double in non-responders (0.41% to 0.87%) (Figure 4). PD1 expression could not be evaluated at this point due to receptor occupancy by Pembrolizumab and no significant changes were observed in PDL1 expression in monocytes, dendritic or plasma cells.
Conclusions
This first evaluation of Pembrolizumab monotherapy as consolidation in MM shows that it is well tolerated and is able to upgrade the response in some patients. However, early progressions, which are associated with lower basal NK numbers and a lower PD1 expression in effector memory CD8 cells, may be of concern. Further analysis in the whole population will be presented at the meeting.
This study rece ive financial support from Merck Sharp & Dohme of Spain, a subsidiary of Merck & Co., Inc., Whitehouse Station, New Jersey, USA
Ocio: Amgen: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy; MSD: Research Funding; Janssen: Honoraria; BMS: Honoraria; Seattle Genetics: Consultancy; Pharmamar: Consultancy; Array Pharmaceuticals: Research Funding; Mundipharma: Research Funding; Celgene: Honoraria, Research Funding. de la Rubia: Amgen: Other: Honoraria; Celgene: Other: Honoraria; Janssen: Other: Honoraria. Oriol: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Celgene: Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia. Paiva: EngMab: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria; Novartis: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria. Lahuerta: Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. San Miguel: Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Mateos: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal